Revolutionary 'Fridge-Free' Vaccine Begins UK Trials
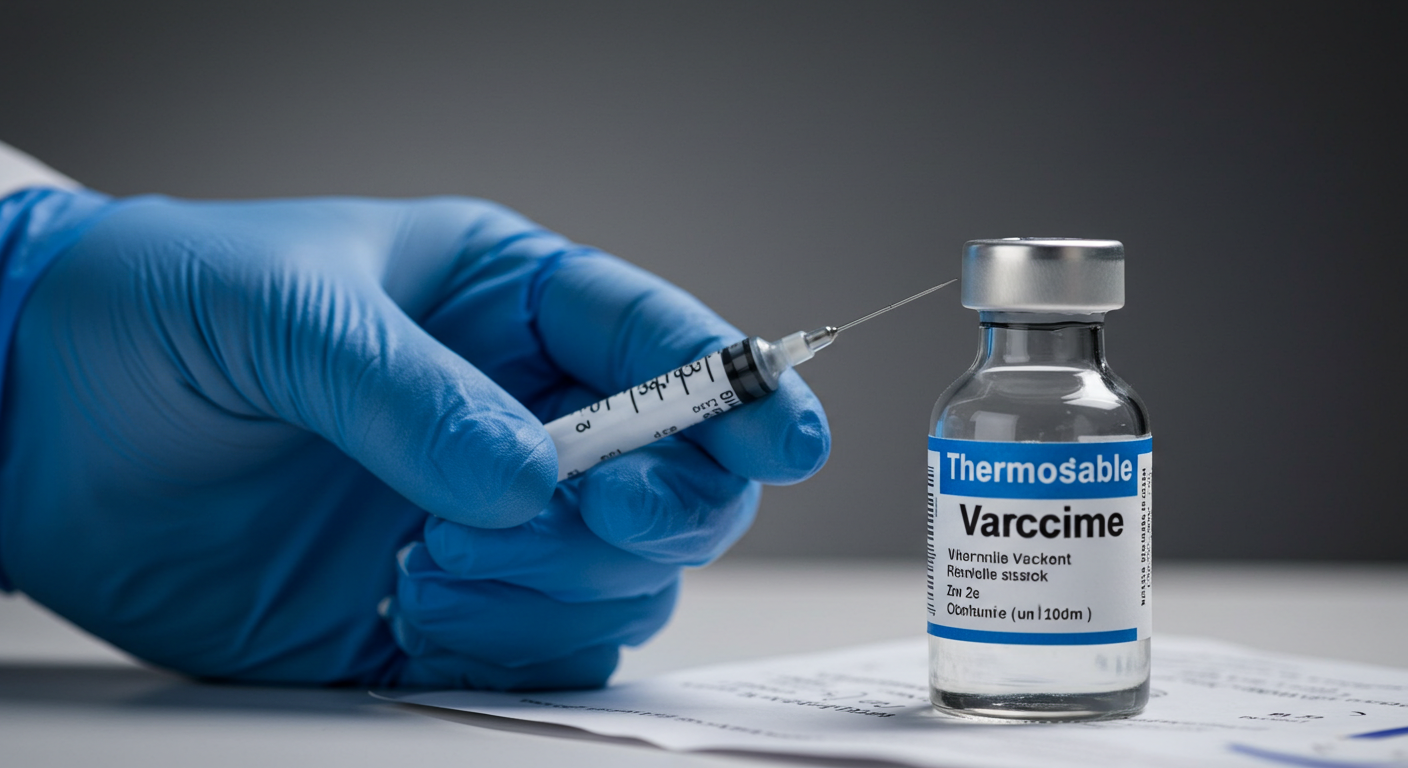
In a groundbreaking development, British scientists have initiated the world's first clinical trial of a "fridge-free" vaccine, potentially transforming global healthcare logistics. Developed by UK biotech firm Stablepharma, this innovative vaccine technology addresses the challenges of maintaining the "cold chain"—the refrigeration process essential for preserving vaccine efficacy.
The thermostable technology converts existing vaccines into versions that remain stable between -20°C and 40°C, allowing storage at room temperature for up to 18 months. This advancement could significantly reduce vaccine wastage, as approximately half of all vaccines are currently discarded annually due to cold chain failures.
The current trial, conducted at University Hospital Southampton, is testing SPVX02, a tetanus-diphtheria vaccine. If successful, this technology could be deployed globally by 2027, enhancing vaccine access in remote or underdeveloped regions. Stablepharma has identified 60 potential vaccines for future development and possesses the capacity to manufacture millions of doses annually.
Supported by the UK government and the National Institute for Health and Care Research (NIHR), this initiative is seen as a transformative step in global healthcare, particularly in improving vaccine accessibility and reducing waste.
Conclusion:
The successful development and deployment of thermostable vaccines could revolutionize global immunization efforts, especially in areas lacking reliable refrigeration infrastructure. By mitigating cold chain dependencies, this innovation promises to enhance vaccine distribution efficiency, reduce waste, and contribute significantly to universal health coverage goals.
Resource:
The Times – 'Revolutionary' fridge-free vaccines to be trialled in UK
Details
Author
Top articles
You can now watch HBO Max for $10
Latest articles
You can now watch HBO Max for $10
In a groundbreaking development, British scientists have initiated the world's first clinical trial of a "fridge-free" vaccine, potentially transforming global healthcare logistics. Developed by UK biotech firm Stablepharma, this innovative vaccine technology addresses the challenges of maintaining the "cold chain"—the refrigeration process essential for preserving vaccine efficacy.
The thermostable technology converts existing vaccines into versions that remain stable between -20°C and 40°C, allowing storage at room temperature for up to 18 months. This advancement could significantly reduce vaccine wastage, as approximately half of all vaccines are currently discarded annually due to cold chain failures.
The current trial, conducted at University Hospital Southampton, is testing SPVX02, a tetanus-diphtheria vaccine. If successful, this technology could be deployed globally by 2027, enhancing vaccine access in remote or underdeveloped regions. Stablepharma has identified 60 potential vaccines for future development and possesses the capacity to manufacture millions of doses annually.
Supported by the UK government and the National Institute for Health and Care Research (NIHR), this initiative is seen as a transformative step in global healthcare, particularly in improving vaccine accessibility and reducing waste.
Conclusion:
The successful development and deployment of thermostable vaccines could revolutionize global immunization efforts, especially in areas lacking reliable refrigeration infrastructure. By mitigating cold chain dependencies, this innovation promises to enhance vaccine distribution efficiency, reduce waste, and contribute significantly to universal health coverage goals.
Resource:
The Times – 'Revolutionary' fridge-free vaccines to be trialled in UK
Top articles
You can now watch HBO Max for $10
Latest articles
You can now watch HBO Max for $10
This is a page that only logged-in people can visit. Don't you feel special? Try clicking on a button below to do some things you can't do when you're logged out.
Example modal
At your leisure, please peruse this excerpt from a whale of a tale.
Chapter 1: Loomings.
Call me Ishmael. Some years ago—never mind how long precisely—having little or no money in my purse, and nothing particular to interest me on shore, I thought I would sail about a little and see the watery part of the world. It is a way I have of driving off the spleen and regulating the circulation. Whenever I find myself growing grim about the mouth; whenever it is a damp, drizzly November in my soul; whenever I find myself involuntarily pausing before coffin warehouses, and bringing up the rear of every funeral I meet; and especially whenever my hypos get such an upper hand of me, that it requires a strong moral principle to prevent me from deliberately stepping into the street, and methodically knocking people's hats off—then, I account it high time to get to sea as soon as I can. This is my substitute for pistol and ball. With a philosophical flourish Cato throws himself upon his sword; I quietly take to the ship. There is nothing surprising in this. If they but knew it, almost all men in their degree, some time or other, cherish very nearly the same feelings towards the ocean with me.







Comment